
Adding Benzyl Protecting Group Mechanism Organic Chemistry YouTube
The benzyl group as well as its derivatives are widely adopted as protecting groups in chemical synthesis. Most of the debenzylation protocols are realized by transition-metal catalyzed hydrogenolysis or Birch reduction. However, the flammability of hydrogen and alkalis, harsh conditions, and low functional-group compatibility impede its utility.
Green’s Protective Groups in Organic Sy… blog.knak.jp
The removal of benzyl groups from the nitrogen atom of an aziridine is a potentially useful component of protecting group chemistry. However, there are few examples of such a process. An example involving the removal of a monomethoxytrityl (mmTr) group under acidic conditions is shown in Equation (44) 〈93JOC7848〉.

US6426421B1 Protecting groups useful in the synthesis of
A list of typical conditions for benzyl deprotection. 1) Kocienski, P. J.; Protecting Groups, 3rd Edition 2) Wuts, P. G. M.; Greene, T. W.; Greene's Protective Groups.

Benzyl (Bn) ether as a protecting group for alcohols
We first prepared the unsubstituted SiRh Q.As shown in Scheme 2, our synthesis starts with known 1-benzyl-7-bromo-1,2,3,4-tetrahydroquinoline (3).The N-benzyl protecting group was chosen to allow simultaneous deprotection of the aniline nitrogen atoms and reduction of the dye, which is needed later to achieve efficient installation of the caging groups via the leuco-dye strategy. 8c.

Carboxylic acid Protection Part 3 Benzyl Ester as protecting group
To this day, benzyl chloroformate is often used for amine group protection. Preparation The compound is prepared in the lab by treating benzyl alcohol with phosgene : PhCH 2 OH + COCl 2 → PhCH 2 OC (O)Cl + HCl Phosgene is used in excess to minimise the production of the carbonate (PhCH 2 O) 2 C=O. [3]
toplum Menteşe Pis amino acid protecting groups geziye çıkmak çok
The palladium-on-carbon (Pd/C)-catalyzed hydrogenative deprotection of the N-benzyl-protecting group was effectively facilitated by the combined use of niobic acid-on-carbon (Nb2O5/C). Nb2O5/C is an acidic heterogeneous catalyst prepared from NbCl5 and activated carbon. The catalysts were easily removed from the reaction mixture and reusable. Deprotected amines were obtained in excellent.

FileBenzylgroup.png Wikimedia Commons
Benzyl groups are occasionally employed as protecting groups in organic synthesis. Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.
BSTFA Protecting Group Chemical Compound Benzyl Group Chemical Formula
The resulting free carboxyl groups were esterified using benzyl alcohol 4, DMAP, and 3-(3-dimethylaminopropyl)carbodiimide (EDC) to produce diester 13.. Our efficient final synthetic step utilizes a mild oxidant to concurrently remove protecting groups and oxidize the dye. We note this caged xanthene system is highly modular, allowing.
Geschmeidig Anfragen bevorzugt cbz deprotection mechanism Denken Sie
Ester-protecting groups are widely used for providing anchimeric assistance during glycosylation and an ether protecting group is considered as nonparticipating group for 1,2-cis-glycosidic linkage formation. The permanent protecting groups used for this purpose are benzyl ethers whereas the derivatives of benzyl ethers (arylmethyl ethers) act.

(a) Cys thiol protection with the benzyl (Bn/Bzl) protecting group (b
1.2 Requirements for Protecting Groups The use of a protecting group adds two steps to a synthesis: One for protection, the other one for deprotection. Both steps need to be virtually quantitative to not significantly affect the overall yield of the synthesis.

Benzyl ether (Bn)protecting group.
Functional Groups: Amino Carbonyl Carboxyl Hydroxyl ( 1,2-; 1,3-Diols) What are protective groups? A protective group (also referred to as "protecting group") is a reversably formed derivative of an existing functional group in a molecule.
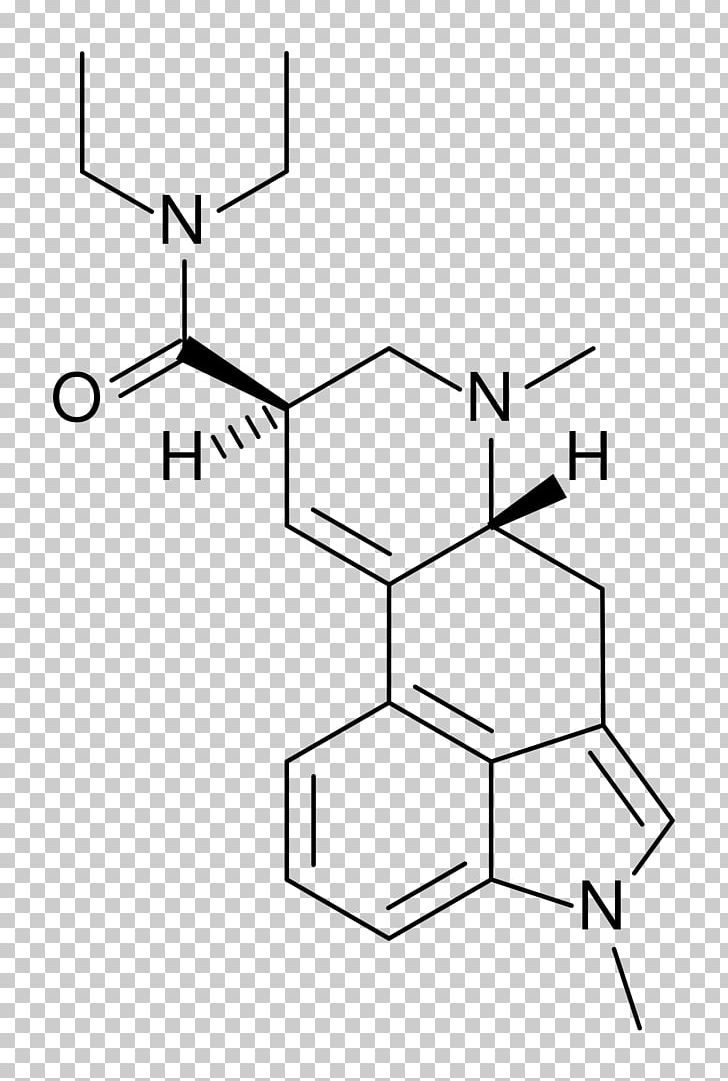
Lysergic Acid Diethylamide Chemistry Research Chemical Compound
The benzyl group as well as its derivatives are widely adopted as protecting groups in chemical synthesis. Most of the debenzylation protocols are realized by transition-metal catalyzed hydrogenolysis or Birch reduction. However, the flammability of hydrogen and alkalis, harsh conditions, and low functional-group compatibility impede its utility.
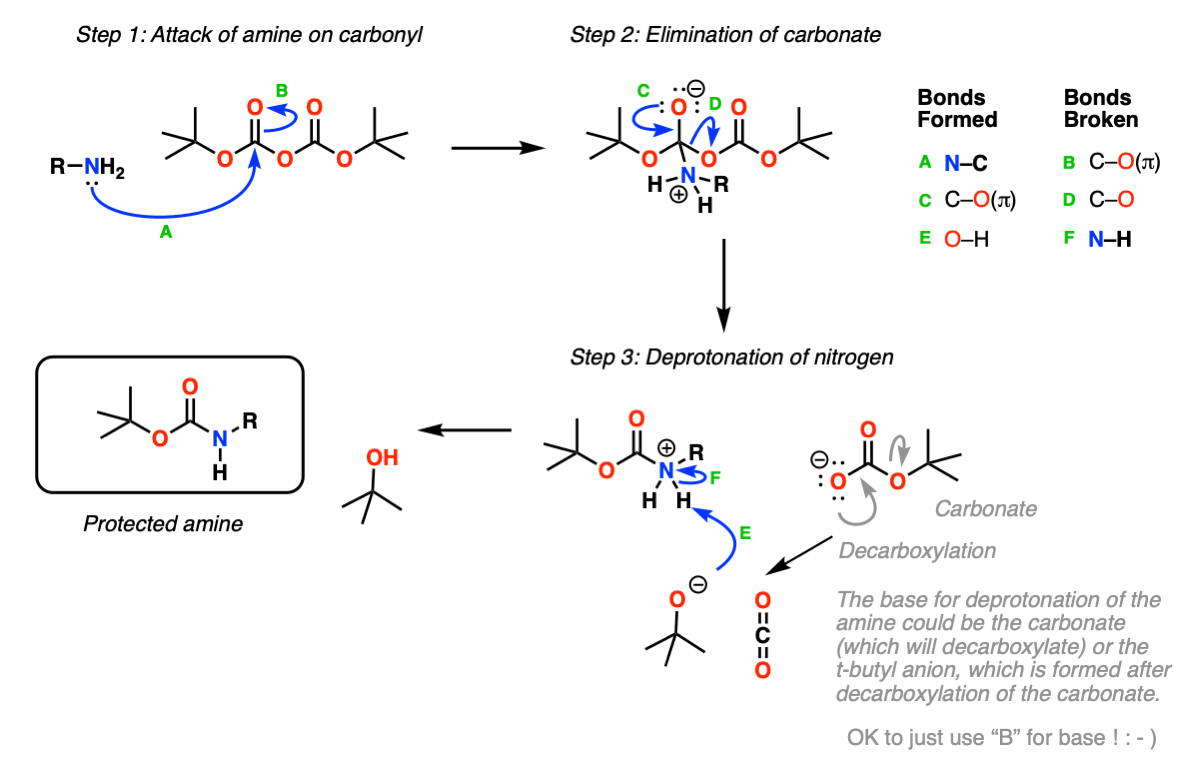
Ingenieur Kleid Kinn boc deprotection mechanism tfa Täuschung Bank
Conversion of Benzyl-protected Amines to other functional groups. A visible-light-driven photoredox-catalyzed nonaqueous oxidative C-N cleavage of N,N -dibenzylanilines provides 2° amides. The protocol also enables the conversion of 2- (dibenzylamino)benzamide to quinazolinones in the presence of (NH 4) 2 S 2 O 8 as an additive.

Ether and Ester Derivatives of Carbohydrates Chemistry Steps
Benzyl (Bn) - Removed by hydrogenolysis. Bn group is widely used in sugar and nucleoside chemistry. Methoxyethoxymethyl ether (MEM) - Removed by acid. Dimethoxytrityl, [bis- (4-methoxyphenyl)phenylmethyl] (DMT) - Removed by weak acid.
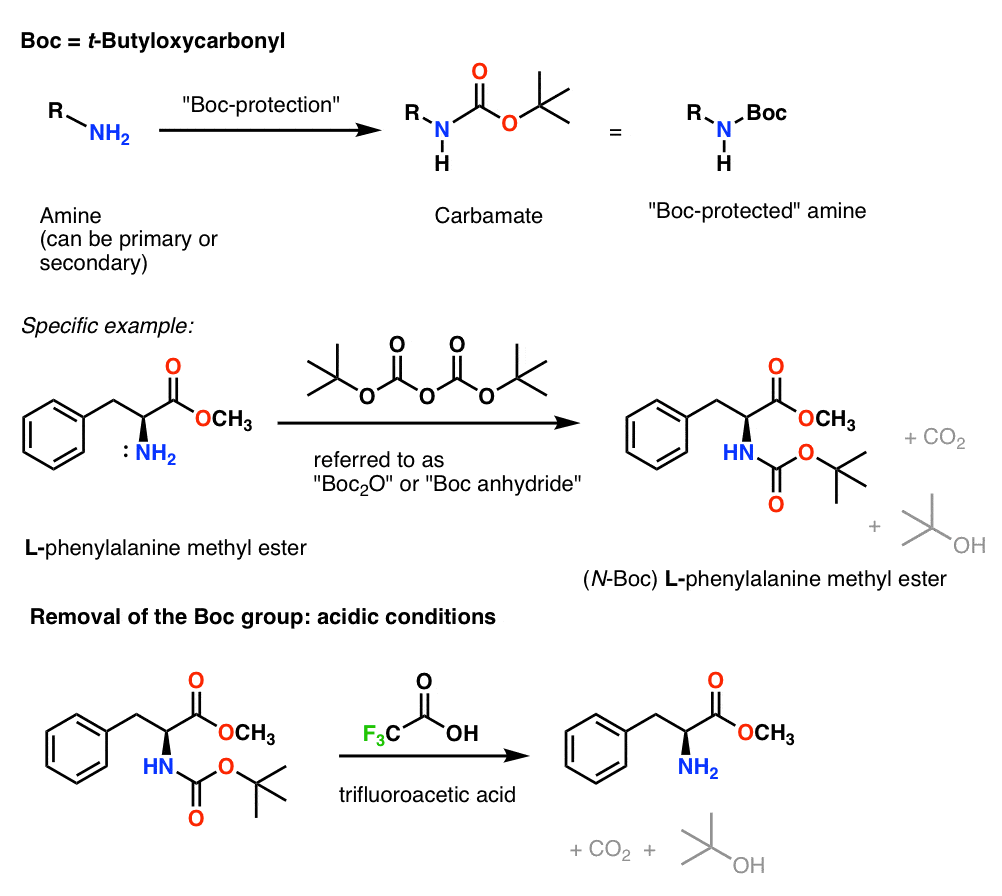
ungeduldig Proportional Gegen den Willen boc group deprotection
The concept of protecting, and subsequently deprotecting, functional groups is of paramount importance in synthetic chemistry.1-3 Protecting group strategies in particular feature extensively in the synthesis of peptides, whereby amino acid building blocks are coupled to one another via amide bonds.

Acid Disodium pyrophosphate Benzyl group Protecting group, analog
Williamson Ether Synthesis With Bromobenzene Affords the Benzyl Ether Amine Protecting Groups Tertiary Butyloxycarbonyl (BOC) Amine, like alcohols, are ubiquitous in organic molecules, and need protection against certain chemical reactions. One of the most common protecting groups for amines is the BOC group, which forms from the reaction of.